Benjamin C Tendler, Taylor Hanayik, Olaf Ansorge, Sarah Bangerter-Christensen, Gregory S Berns, Mads F Bertelsen, Katherine L Bryant, Sean Foxley, Martijn P van den Heuvel, Amy FD Howard, Istvan N Huszar, Alexandre A Khrapitchev, Anna Leonte, Paul R Manger, Ricarda AL Menke, Jeroen Mollink, Duncan Mortimer, Menuka Pallebage-Gamarallage, Lea Roumazeilles, Jerome Sallet, Lianne H Scholtens, Connor Scott, Adele Smart, Martin R Turner, Chaoyue Wang, Saad Jbabdi, Rogier B Mars, Karla L Miller
Wellcome Centre for Integrative Neuroimaging, FMRIB, Nuffield Department of Clinical Neurosciences, University of Oxford, United Kingdom; Division of Clinical Neurology, Nuffield Department of Clinical Neurosciences, University of Oxford, United Kingdom; Psychology Department, Emory University, United States; Centre for Zoo and Wild Animal Health, Copenhagen Zoo, Denmark; Department of Radiology, University of Chicago, United States; Department of Complex Trait Genetics, Centre for Neurogenomics and Cognitive Research, Amsterdam Neuroscience, Vrije Universiteit Amsterdam, Netherlands; Department of Child Psychiatry, Amsterdam Neuroscience, Amsterdam UMC, Vrije Universiteit Amsterdam, Netherlands; Medical Research Council Oxford Institute for Radiation Oncology, University of Oxford, United Kingdom; School of Anatomical Sciences, Faculty of Health Sciences, University of the Witwatersrand, South Africa; Wellcome Centre for Integrative Neuroimaging, Department of Experimental Psychology, University of Oxford, United Kingdom; Stem Cell and Brain Research Institute, Université Lyon 1, INSERM, France; Donders Institute for Brain, Cognition and Behaviour, Radboud University Nijmegen, Netherlands
Abstract
Post-mortem magnetic resonance imaging (MRI) provides the opportunity to acquire high-resolution datasets to investigate neuroanatomy and validate the origins of image contrast through microscopy comparisons. We introduce the Digital Brain Bank (open.win.ox.ac.uk/DigitalBrainBank), a data release platform providing open access to curated, multimodal post-mortem neuroimaging datasets. Datasets span three themes—Digital Neuroanatomist: datasets for detailed neuroanatomical investigations; Digital Brain Zoo: datasets for comparative neuroanatomy; and Digital Pathologist: datasets for neuropathology investigations. The first Digital Brain Bank data release includes 21 distinctive whole-brain diffusion MRI datasets for structural connectivity investigations, alongside microscopy and complementary MRI modalities. This includes one of the highest-resolution whole-brain human diffusion MRI datasets ever acquired, whole-brain diffusion MRI in fourteen nonhuman primate species, and one of the largest post-mortem whole-brain cohort imaging studies in neurodegeneration. The Digital Brain Bank is the culmination of our lab’s investment into post-mortem MRI methodology and MRI-microscopy analysis techniques. This manuscript provides a detailed overview of our work with post-mortem imaging to date, including the development of diffusion MRI methods to image large post-mortem samples, including whole, human brains. Taken together, the Digital Brain Bank provides cross-scale, cross-species datasets facilitating the incorporation of post-mortem data into neuroimaging studies.
死後脳(post-mortem brain)の磁気共鳴画像(MRI)は、神経解剖学の調査や顕微鏡比較による画像コントラストの起源を検証するための高解像度データセットを取得する機会を提供する。デジタル・ブレイン・バンク (open.win.ox.ac.uk/DigitalBrainBank) は、マルチモーダルな死後神経画像データセットにオープンアクセスするためのデータ公開プラットフォームである。データセットは、Digital Neuroanatomist:詳細な神経解剖学的調査のためのデータセット、Digital Brain Zoo:比較神経解剖学のためのデータセット、Digital Pathologist:神経病理学的調査のためのデータセットの3つのテーマに分かれている。デジタル・ブレインバンクの最初のデータリリースには、顕微鏡画像や補完的なMRIモダリティに加え、構造的結合を調べるための21の特徴的な全脳拡散MRIデータセットが含まれている。この中には、これまでに取得された最も高解像度のヒト全脳拡散MRIデータセット、14種の霊長類の全脳拡散MRI、神経変性における最大規模の死後全脳コホート画像研究の1つが含まれています。デジタル・ブレインバンクは、死後脳MRIの方法論とMRI-顕微鏡解析技術に対する我々の研究室の投資の集大成である。この原稿では、ヒトの全脳を含む大きな死後サンプルの画像を得るための拡散MRI法の開発など、死後画像に関する我々のこれまでの研究の詳細な概要を説明しています。デジタル・ブレインバンクは、神経イメージング研究に死後データを取り入れることを容易にする、クロススケール、種間比較のデータセットを提供する。
Digital-Brain-Bank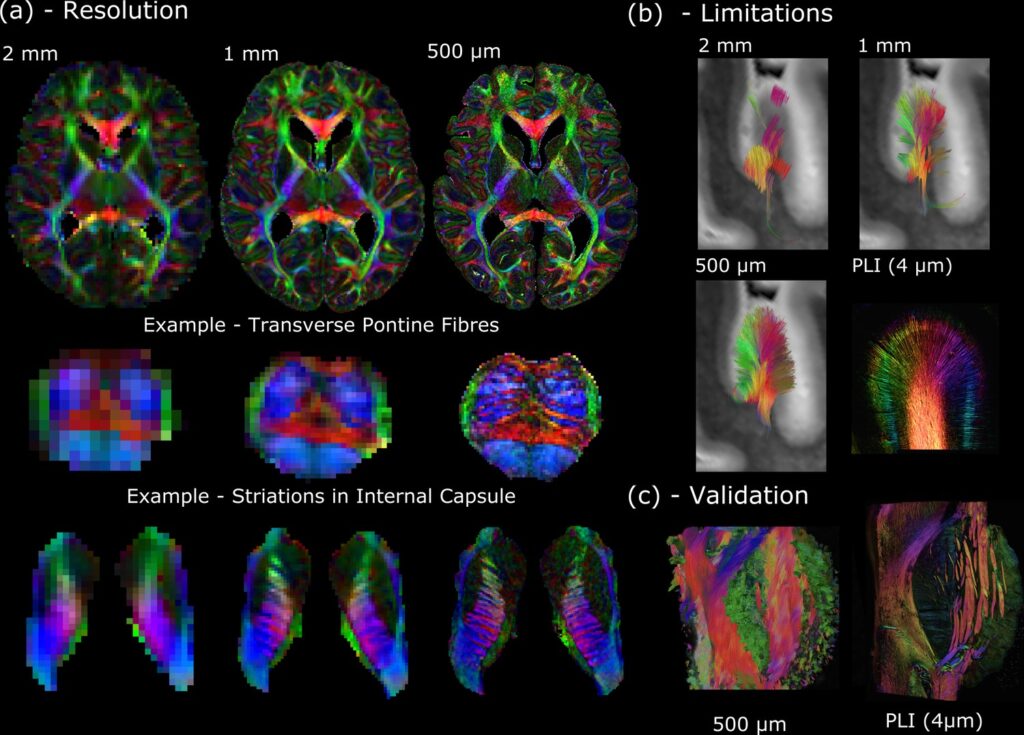
(a) Whole-brain diffusion MRI data available in the Human High-Resolution Diffusion MRI-PLI dataset reveals the wealth of information provided at increased spatial scales, one of the key aims of the Digital Anatomist. Here, the 500 μm dataset uncovers the information hidden at lower spatial resolutions, for example, visualizing the interdigitating transverse pontine fibers with the corticospinal tract or striations through the internal capsule. (b) Similarly, datasets across multiple spatial scales can inform us of the limitations at reduced imaging resolutions. Here, gyral tractography (occipital lobe) reveals an overall pattern of fibers turning into the gyral bank at 0.5 mm. At 1 mm, an underestimation of connectivity at the gyral banks is observed, known as the ‘gyral bias’ (Cottaar et al., 2021; Schilling et al., 2018). At 2 mm, tractography bears little resemblance to the expected architecture. Multimodal comparisons enable us to validate our findings, with complementary polarised light imaging (PLI) data at over 2 orders of magnitude increase in resolution (125×) revealing a similar pattern of gyral connectivity, and (c) excellent visual agreement with tractography across the pons. (a) displays diffusion tensor principal diffusion direction maps (modulated by fractional anisotropy).
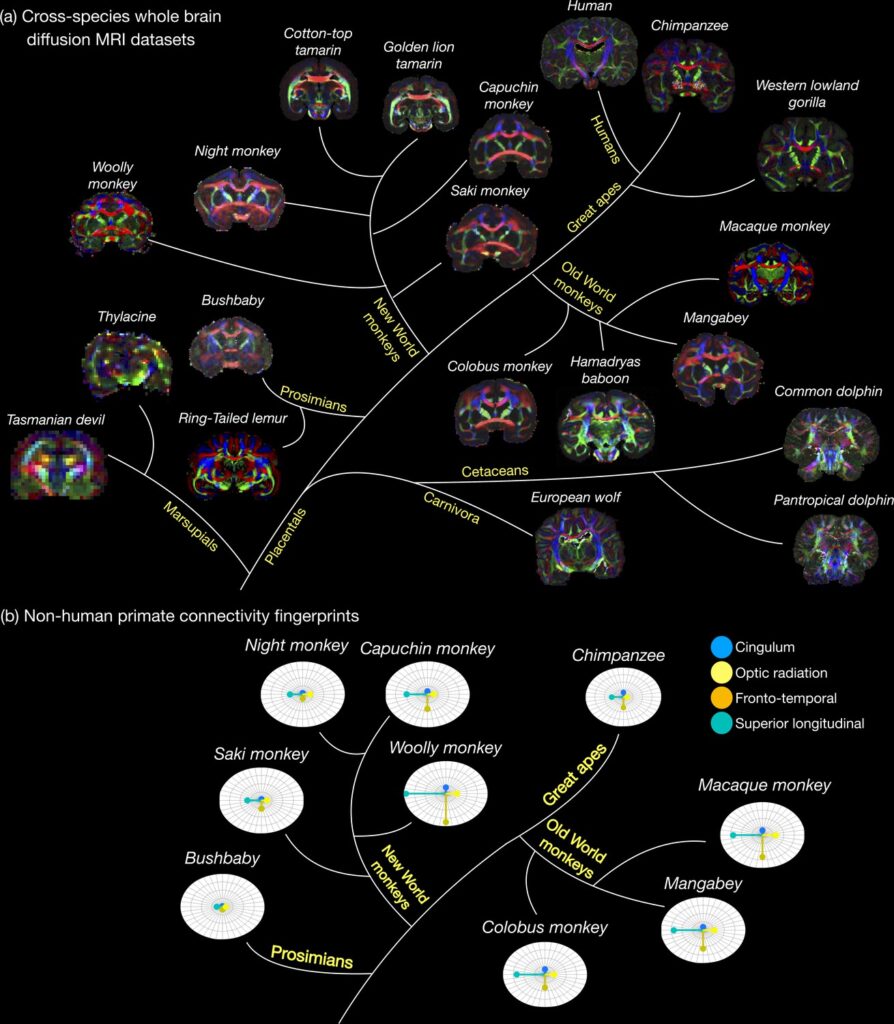
(a) The first release of the Digital Brain Zoo provides whole-brain MRI datasets spanning multiple species and taxonomic ranks. Notably, we provide whole-brain diffusion MRI datasets from 14 nonhuman primate species, with samples selected for their high quality and to ensure sampling of all major branches of the primate evolutionary tree (Prosimian, New World monkey, Old World monkey, and Great Ape). (b) compares the relative volume of four tracts derived from nine nonhuman primate post-mortem datasets provided in the Digital Brain Zoo (Bryant et al., 2021), where increased distance from the centre corresponds to an increased volume.
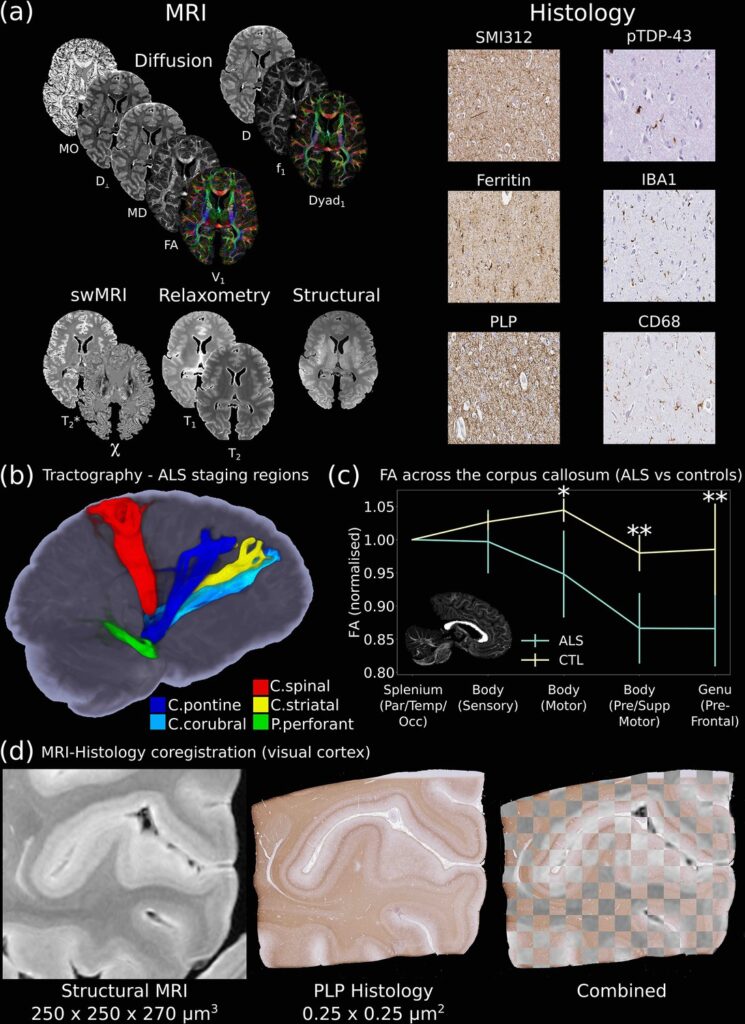
One of the key aims of the Digital Pathologist is the examination of neuropathological spread in neurological disease. The Human ALS MRI-Histology dataset (a) facilitates these investigations, combining whole-brain multimodal MRI and histology (selected brain regions) in a cohort of 12 ALS and 3 control brains. (b) Displays the reconstruction of five white matter pathways associated with different ALS stages in a single post-mortem brain (Kassubek et al., 2014). Comparisons between ALS and control brains over the corpus callosum of the cohort (c) reveals changes in fractional anisotropy (FA, normalized to Par/Temp/Occ lobe), with biggest changes associated with motor and prefrontal regions (Hofer and Frahm, 2006) (*=p<0.05; **=p<0.05 following multiple comparison correction) (full details of the corpus callosum analysis provided in Appendix 2). This reflects the anticipated changes in ALS with brain regions associated with motor function, in good agreement with a previous study (Chapman et al., 2014), which identified the greatest FA difference between ALS and controls in these regions. Accurate MRI-histology coregistrations facilitate cross-modality comparisons, and (d) displays an example of MRI-histology coregistration over the visual cortex of a single ALS brain achieved using the Tensor Image Registration Library (TIRL) (Huszar et al., 2019). V1=principal diffusion direction, FA=fractional anisotropy, MD=mean diffusivity, D⊥=radial diffusivity, MO=mode from diffusion tensor output, Dyad1=principal dyad orientation, f1=principal fiber fraction and D=diffusivity from Ball and Two-Stick output, swMRI=susceptibility-weighted MRI, χ=magnetic susceptibility. Details of stain contrasts in (b) and (d) are provided in Table 1. ALS, amyotrophic lateral sclerosis; MRI, magnetic resonance imaging.
