SCIENCE IMMUNOLOGY 11 Oct 2019 Vol 4, Issue 40 DOI: 10.1126/sciimmunol.aay5199
RESEARCH ARTICLENEUROIMMUNOLOGY
Miguel Ribeiro 1, Helena C Brigas 1, Mariana Temido-Ferreira 1, Paula A Pousinha 2, Tommy Regen 3, Cátia Santa 4 5, Joana E Coelho 1, Inês Marques-Morgado 1, Cláudia A Valente 1, Sara Omenetti 6, Brigitta Stockinger 6, Ari Waisman 3, Bruno Manadas 4, Luísa V Lopes 1, Bruno Silva-Santos
Instituto de Medicina Molecular João Lobo Antunes, Faculdade de Medicina, Universidade de Lisboa, Lisboa, Portugal.
T cells that promote memories
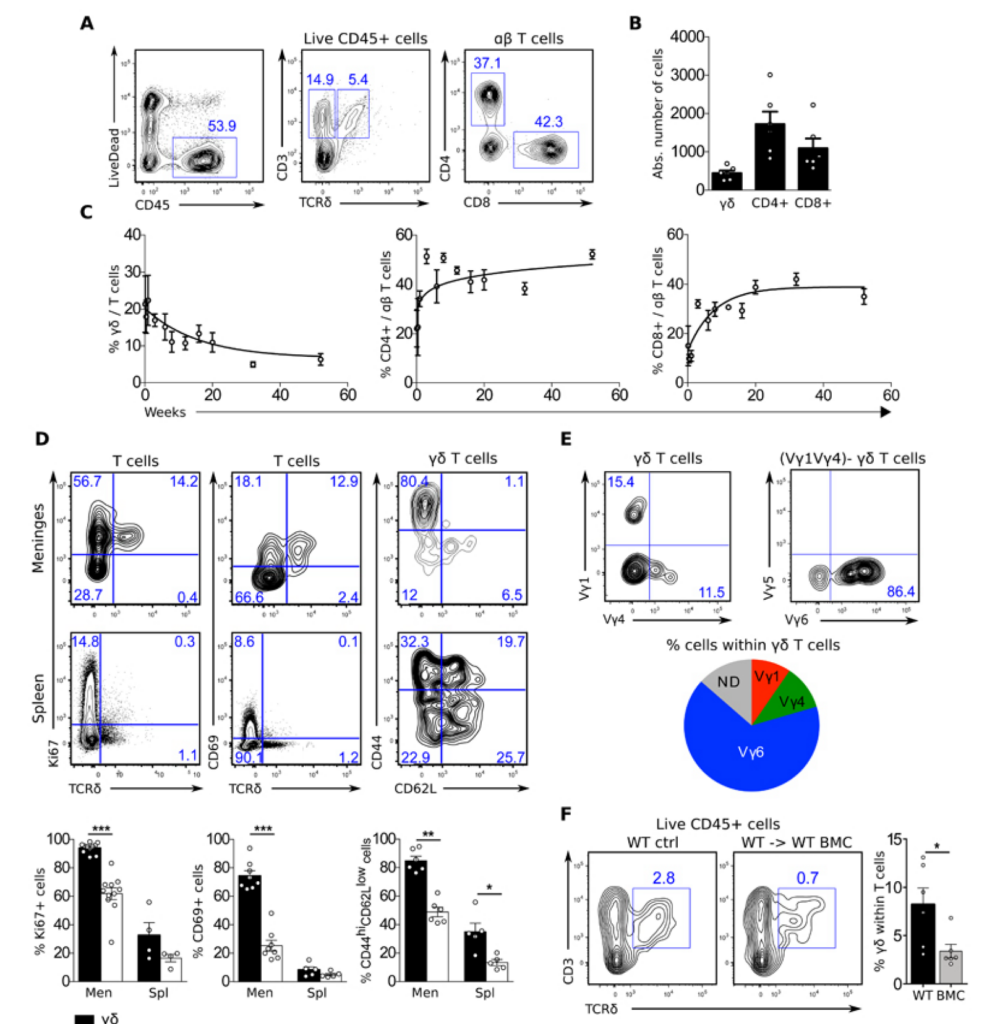
There is now a growing appreciation of cross-talk between the nervous and immune systems. Here, Ribeiro et al. report that interleukin-17 (IL-17)–producing γδ T cells that arise early in life and home to the meninges of neonatal mice play an essential role in the development of short-term memories. Both mice lacking γδ T cells and IL-17 show impaired performance in Y-maze tests that rely on short-term memory but did not show any deficits in other behavioral tests, including tests that gauge long-term memory. The authors found that meningeal IL-17–producing γδ T cells promote secretion of brain-derived neurotropic factor in mouse brains. Further studies are needed to understand the local and systemic effects of IL-17 on neuronal and non-neuronal cells in the brain.
記憶を促進するT細胞
神経系と免疫系の間のクロストークについて、現在、評価が高まってきている。今回、Ribeiroらは、新生児マウスの髄膜で早期に発生するインターロイキン17(IL-17)産生γδT細胞が、短期記憶の形成に必須な役割を果たすことを報告した。γδT細胞とIL-17を欠損したマウスは、いずれも短期記憶に依存するY迷路テストの成績に障害を示したが、長期記憶を測定するテストを含む他の行動テストでは障害を示さなかった。筆者らは、髄膜のIL-17産生γδT細胞が、マウス脳内の脳由来神経栄養因子の分泌を促進することを明らかにした。脳の神経細胞および非神経細胞に対するIL-17の局所的および全身的な影響を理解するために、さらなる研究が必要である。
Abstract
The notion of “immune privilege” of the brain has been revised to accommodate its infiltration, at steady state, by immune cells that participate in normal neurophysiology. However, the immune mechanisms that regulate learning and memory remain poorly understood. Here, we show that noninflammatory interleukin-17 (IL-17) derived from a previously unknown fetal-derived meningeal-resident γδ T cell subset promotes cognition. When tested in classical spatial learning paradigms, mice lacking γδ T cells or IL-17 displayed deficient short-term memory while retaining long-term memory. The plasticity of glutamatergic synapses was reduced in the absence of IL-17, resulting in impaired long-term potentiation in the hippocampus. Conversely, IL-17 enhanced glial cell production of brain-derived neurotropic factor, whose exogenous provision rescued the synaptic and behavioral phenotypes of IL-17–deficient animals. Together, our work provides previously unknown clues on the mechanisms that regulate short-term versus long-term memory and on the evolutionary and functional link between the immune and nervous systems.