Glia
Open Access
Transnasal transplantation of human induced pluripotent stem cell-derived microglia to the brain of immunocompetent mice (ヒト iPS 細胞由来ミクログリアの完全非侵襲的なマウス脳移植法)
Bijay Parajuli, Hiroki Saito, Youichi Shinozaki, Eiji Shigetomi, Hiroto Miwa, Sosuke Yoneda, Miki Tanimura, Shigeki Omachi, Toshiyuki Asaki, Koji Takahashi, Masahide Fujita, Kinichi Nakashima, Schuichi Koizumi
First published: 26 July 2021 https://doi.org/10.1002/glia.23985
Abstract
Microglia are the resident immune cells of the brain, and play essential roles in neuronal development, homeostatic function, and neurodegenerative disease. Human microglia are relatively different from mouse microglia. However, most research on human microglia is performed in vitro, which does not accurately represent microglia characteristics under in vivo conditions. To elucidate the in vivo characteristics of human microglia, methods have been developed to generate and transplant induced pluripotent or embryonic stem cell-derived human microglia into neonatal or adult mouse brains. However, its widespread use remains limited by the technical difficulties of generating human microglia, as well as the need to use immune-deficient mice and conduct invasive surgeries. To address these issues, we developed a simplified method to generate induced pluripotent stem cell-derived human microglia and transplant them into the brain via a transnasal route in immunocompetent mice, in combination with a colony stimulating factor 1 receptor antagonist. We found that human microglia were able to migrate through the cribriform plate to different regions of the brain, proliferate, and become the dominant microglia in a region-specific manner by occupying the vacant niche when exogenous human cytokine is administered, for at least 60 days.
ミクログリアは脳の常在免疫細胞であり、神経発生、恒常性機能、神経変性疾患において重要な役割を果たしている。ヒトミクログリアはマウスミクログリアとは性質がかなり異なる。ヒトのミクログリアに関するほとんどの研究はin vitroで行われ、in vivo条件下でのミクログリアの特性を正確に表してはいない。ヒトミクログリアのin vivo特性を解明するために、人工多能性幹細胞または胚性幹細胞由来のヒトミクログリアを生成し、新生児または成体のマウスの脳に移植する方法が開発されている。しかし、ヒトのミクログリアを生成する技術的な難しさ、および免疫不全マウスを使用して侵襲的な手術を行う必要性によって、その利用は制限されたままである。これらの問題に対処するために、筆者たち(山梨大学医学部薬理学研究室の小泉修一教授らのグループ)は、ヒト人工多能性幹細胞(iPS細胞)由来のミクログリアを作成し、コロニー刺激因子1受容体(CSF1R)拮抗薬と組み合わせることで、免疫能の正常なマウスの経鼻経路を介して脳に移植する簡単な方法を開発しました。ヒトミクログリアは、篩板を通って脳のさまざまな領域に移動し、増殖した。外因性のヒトサイトカインを少なくとも60日間投与すると、マウス脳内で空のニッチを占めることにより、領域特異的な方法で優勢なミクログリアになることができることを見出した 。本研究により、ミクログリアが関係する種々の脳疾患、老化の仕組みがヒトミクログリアを使ったin vivo研究によって明らかになること、ミクログリア移植による新しい細胞治療法が開発されることが期待される。
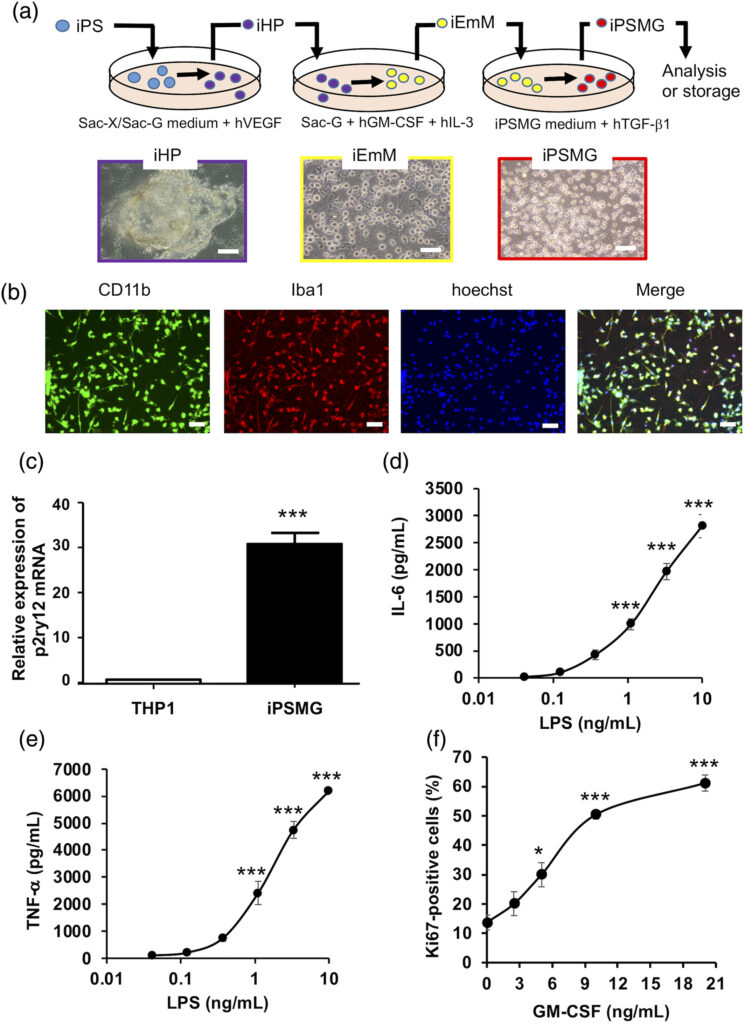
FIGURE 1
Generation and characterization of iPSC-derived human microglia (iPSMG). (a) Schematic showing the process of differentiation from iPSCs, through iHP (0–13 days) and iEmM (13–19 days), to iPSMG (19–27 days). iPSCs seeded on 10T1/2 cells were induced to undergo differentiation to iHP cells. Differentiated iHP cells were observed in sac-like structures (left photo). The iHP cells were then transferred to new medium and seeded onto 10T1/2 cells for 6 days to induce iEmM. Differentiated iEmM are shown in the middle photo. Finally, iEmM cells were transferred to new medium and seeded onto rat astrocytes for 9 days to induce microglial differentiation. The last 3 days of iPSMG differentiation before the other experiments included the addition of hTGF-β1. Differentiated iEmM are shown in the right photo. Scale bars = 100 μm. (b) Purity of iPSMG were tested by immunostaining by CD11b and Iba1. iPSMG were fixed and immunostained for CD11b (red) and Iba1 (green). Cell nuclei were counterstained with Hoechst 33342 (blue). Merged image of CD11b, Iba1 and Hoechst 33342 demonstrated the purity of iPSMG culture close to 100%. Photographs show one representative image from five independent experiments. Scale bars = 100 μm. (c) The mRNA expression of P2RY12 was assessed by RT-PCR. iPSMG had higher expression of P2RY12 than THP1 cells. ***p < .001, versus THP1 cells (unpaired t-test). (d) The secretion of IL-6 was measured after activation with LPS. LPS stimulation increased secretion of IL-6 compared to untreated control. Data represents mean ± SEM of 5 independent experiments. ***p < 0.001, versus untreated control (one-way ANOVA with Tukey’s multiple comparison test). (e) The secretion of TNF-α was measured after activation with LPS. LPS stimulation increased secretion of TNF-α compared to untreated control. Data represents mean ± SEM of five independent experiments. ***p < 0.001, versus untreated control (one-way ANOVA with Tukey’s multiple comparison test). (f) iPSMG proliferation was assessed by immunostaining with Ki67 after treatment with varying doses of GM-CSF. GM-CSF treatment dose-dependently increased iPSMG proliferation. Data represents mean ± SEM of 5 independent experiments. *p < 0.05, ***p < 0.001, versus untreated control (one-way ANOVA with Tukey’s multiple comparison test)
日本の研究.com ヒト iPS 細胞由来ミクログリアの完全非侵襲的な脳移植法の開発に成功 -脳疾患の病因解明と新規細胞治療法に期待